Introduction
Here is an introduction video that sets up the tone for this blog: Basically, NITROGEN = LIFE
Here is an introduction video that sets up the tone for this blog: Basically, NITROGEN = LIFE
Throughout the history, human activities have had
significant impacts on the Nitrogen cycle. Activities such as burning fossil
fuels, utilization of Nitrogen-based fertilization, and other activities have
lead to an increase in the total amount of biousable Nitrogen in ecosystems
globally. Nitrogen availability is directly related to primary production in
many terrestrial and aquatic ecosystems; thus, large changes in the
availability of Nitrogen can result in extreme alterations in the Nitrogen
cycle. Vitousek et al. 1997 (Vitousek,
Mooney, Lubchenco, & Melillo, 1997) , discusses the
implication of industrial Nitrogen fixation and how as a result of human
activity, total amount of Nitrogen fixation has doubled since the 1940s, on a
global scale.
Located at the 7th position on the
periodic table of elements; a colorless, odorless, and tasteless gas, Nitrogen
is a critical primary nutrient, essential for life to proceed for all living
organisms(Lide &
Milne, 1995). Nitrogen was
discovered in 1772 by Daniel Rutherford. By volume, Nitrogen fills 78% of air
in earth’s atmosphere, compared to Mars, which this percentage is much lower,
2.6%. Regardless of its total contribution to the atmospheric air, its presence
illustrates the important role it plays in the universe as a whole. Despite its
massive abundance in earth’s atmosphere, in its natural form, Nitrogen is not
biousable for many organisms, making it a scare resource and a limiting factor
in many ecosystems globally. Farmers for
instance, need to provide Nitrogen for their crops to maintain growth and
expand economical trades. But how does
this Nitrogen become available to organisms and what happens to Nitrogen levels
as an outcome of human activities, is explored in this blog.
Nitrogen has many uses. Probably the coolest usage
of nitrogen is in the making of light bulbs. An insert gas, nitrogen, is often
used in light bulbs because it fills the light bulb because it prevents air
from entering the light bulb – air and light bulb don’t work well together! Additionally,
physical properties of nitrogen help to conduct energy, in the form of heat,
from the glowing filaments to the glass bulb, Similar to a cargo if you imagine.
Image illustrating the usage of nitrogen as an inert gas in the making of light bulbs. In the old days they used to have a vacuum that sucked air out, but it was too much of a hassle and the lights bulbs were not as bright.
Another usage of nitrogen gas, which had lead to
great discoveries in biological sciences, especially virology, is the usage of
nitrogen gas in cryogenic electron microscopy, or cryo-EM. Regular electron
microscopy has been aiding structural biologist in determining the 3D
structures of various viruses and their proteins (Baker, Olson,
& Fuller, 1999). Through these
studies, many vaccines have been developed. However, regular EM often
introduces bias information due to crystallization of the solvent. With the
usage of liquid nitrogen, which exists at extremely low temperatures (-350-400
C), the samples used in cryo-EM are frozen so fast that the solvent, water in
many cases, does not crystallize and therefore both the sample and the solvent
stay in their most native forms. Discovery of nitrogen in this process has been
a huge breakthrough in virology and cryo-EM.
Left - A 5 million Dollar worth cryogenic electron microscope - Right, The structure of gp140 - a protein on the surface of HIV.
In my opinion, not all uses of nitrogen are
beneficial to humans; and I’m not talking about plants, I’m talking about
BOMBS. Nitrogen triiodide for instance, an extremely sensitive explosive – can be
set off by slightest contact. Even small amounts of nitrogen triiodide can
generate massive energy in the form of sound (Boopathy
& Kulpa, 1992). The trait that
allows nitrogen to be suitable for explosives is that it is not radioactive,
like Uranium for instance, and most of its explosive properties are triggered
through chemical reactions. This is due to its stability and occurrence in only
two isotopes, N14 and N-15.
Image showing the classic TNT explosives as we all remember it in Wiley Coyote. A very sensitive substance that through chemical reaction releases a massive amount of energy.
Before moving onto the natural processes of nitrogen
and humans impacts, I’d like to discuss one last usage of nitrogen, loved by
millions worldwide; the laughing gas!
Perhaps one of the things that make getting your
teeth fixed is the usage of nitrogen in laughing gas. Nitrogen oxide or
dinitrogen oxide is a colorless gas with a sweet smell and taste. Once inhaled,
it causes disorientation euphoria, numbness and loss of motor coordination; in
other words, it knocks you out and the best part is you’ll be laughing while it
happens.
Now let’s move into the biological and
ecological side of Nitrogen:
Nitrogen undergoes a number of alterations and
exists in more than one form; each organism, depending on the nature of the
ecosystem utilizes Nitrogen in fundamentally different forms. Nitrogen is
mainly usable when it is converted from its natural form, N2 (dinitrogen
gas) into Ammonia (NH3), which is used by primary producers.
Nevertheless, Nitrogen exists in a number of other forms as well, either
organic such as in amino acids, or nucleic acids, or in inorganic forms such as
Ammonia and nitrate. Through this cycle, different forms of Nitrogen are used
as nutrients for growth and energy by different living organisms. The major
forms of Nitrogen discussed in this blog are the processes or Nitrogen
fixation, nitrification, denitrification, anammox, and ammonification, as well
as the transformation of Nitrogen into oxidation states, which is a critical
step to productivity in the biosphere and extremely dependant on the activities
of microorganisms, such as bacteria and fungi.
The diagram blow depicts the broad picture the Nitrogen
cycle. Each individual stem illustrated in this cycle will be discussed detail.
Perhaps the most studied process in the Nitrogen
cycle is Nitrogen fixation, during which diNitrogen gas, N2, is converted into Ammonia
(NH3). There are two ways that Nitrogen is converted to Ammonia naturally; by
lightning, or by bacteria in plant root nodules .The most common process of Nitrogen
fixation is done through microorganisms such as Nitrogen fixing bacteria and
fungi. Below is the chemical reaction equation for the process of Nitrogen
fixation. 1 unit of atmospheric Nitrogen gas in reduced to generate 2 units of
biologically usable Ammonia and hydrogen gas.
This process is especially important for plant
nutrition, which often involved other organisms. Through a symbiotic relationship,
some bacteria are found predominantly in what’s called the Rhizosphere – the
soil layer in which the plant root is embedded in. Others are decomposers which
derive their nutrient from decaying organic material in the topsoil. Some of
the most well studied microorganisms that fix Nitrogen are Cyanobacteria, Green
sulfur bacteria, Azotobacteraceae, Rhizobia, and Frankia. These bacteria are
often associated with the roots of various trees and shrubs. The bottom line is
that plants require Ammonia to grow better and faster.
Human
Impacts – Nitrogen Fixation
It can be concluded, that Nitrogen is necessary for
farming and agriculture. The importance of Nitrogen in agriculture was finally
understood in the late 1800; Desfosses was the first scientist to observe that Nitrogen
has unique reactivity with certain chemicals. After the importance of Nitrogen
was well understood, the push was towards finding ways to gain more Ammonia to
facilitate agriculture. In fact, one of the main events that lead to huge human
population growth since the early 1900s was new innovations that industrialized
Nitrogen fixation. Early approaches of attaining biousable Nitrogen was in
Chile, where they used Sodium Nitrate, and unstable source of Nitrogen to
produce Ammonia – these resources were rapidly exhausted. It was finally Fritz
Harber, who in 1909, invented the process of industrial implementation of the
reaction of Nitrogen gas, an unusable form of Nitrogen, and hydrogen gas, which
yield 2 moles of Ammonia.

Nature has limited Nitrogen in the atmosphere the
limiting reagent for primary producers for a reason, and manipulation of the
levels of bioavailable Nitrogen will affect many ecosystems. However, due to
environmental effects of the Anthropogenic Nitrogen has introduced excess Nitrogen
to a Nitrogen-limited environment and disrupted natural equilibrium. It has
caused an increase in productivity in ecosystems and as a result biological
life is threatened though different processes; some discussed below.
Human
Population Growth As a Result of the Haber process
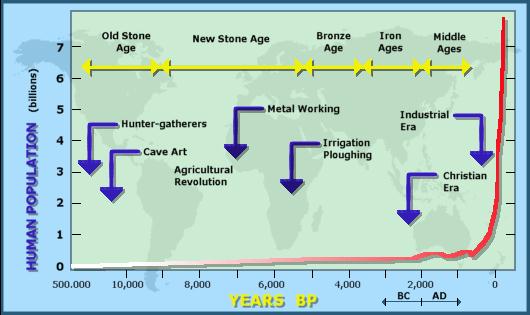
Since the year 1950, we have doubled our
population from three to seven billion(Bloom, 2011) and this number is estimated to
increase to ~10 billion by the year 2050! If it wasn't for major epidemics
throughout human population history (i.e. plague; noted on the graph to the
right), the human population could have been even higher than what it is today.
The figure above illustrates the human growth since the time of pre-agriculture
(Old Stone Age) to present.
The story of nitrogen and human
population growth:
Nitrogen is an essential part of protein
building block, for all amino acid contain a nitrogen group and therefore,
without nitrogen, living organisms cannot sustain life and will die from
proteins deficiency. All living organisms require nitrogen to grow and multiply
in numbers.
People obtain their nitrogen through
their nutrients – by eating green plants, meat, now days, protein bars. The bottom
line is that both animals and plants require nitrogen to sustain life on this
planet. But nitrogen does not come easy! Unfixed nitrogen is as useless to
living organisms as vermiform appendix is to humans. It is the fixed nitrogen
that can be taken up by animal and plant cells and converted into proteins and
amino acids. But how did people figure this out?
Well they didn’t for a long time. Back
at the dawn of civilization, at the beginning of the transformation of
hunter-gatherers to farmers, it was observed by these early humans that crops
such as rice, wheat and corn, only last for a limited amount of time; when
soils got exhausted and depleted of nitrogen, these crops no longer grew (Boopathy
& Kulpa, 1992). It was the legumes that shed
light on the importance of nitrogen and agriculture; the hunter-gatherer people
of course had no idea! Turns out that legume crops such as soy, peas, lentils, alfalfa,
grew much long and iteratively over many years. These crops had roots
associated with a master of nitrogen fixation, rhizobia. This bacterium fixes nitrogen at high rates and makes it
available for plants to take up and utilize the biousable nitrogen. But they
could only fix so much nitrogen in units of metric tons!
Ostensibly, with no excess vegetation globally,
human populations were very small compared to today. It was the Haber-Bosch
process, or Haber process that resulted in the explosion of human population on
earth. Discovered in 1909 as discussed above, the Haber process was able to
produce 300 metric tons of biousable nitrogen annually. The human population
spiked after what’s known as the Green Revolution in the 1940s (Vitousek et
al., 1997). The development or technology in
agriculture between the years 1940s and 1970s, increased agriculture production
on a global scale, particularly in the developing world, leading to massive
increase in world population numbers.
Image of very advanced heavy machinery used in today's agriculture.
Excess
Nitrogen and Nature
Nature has limited Nitrogen in the atmosphere
resulting in Nitrogen to be the limiting reagent for primary producers. Therefore,
manipulation of the levels of bioavailable Nitrogen will affect ecosystems. Because
of environmental effects caused by anthropogenic activities, Nitrogen is being
introduced in excess to a Nitrogen-limited environment which leads to the disruption
of natural Nitrogen equilibrium. This excess Nitrogen has caused an increase in
productivity in ecosystems and as a result biological life is threatened though
different processes; some discussed below.
One of the ways by which release of Anthropogenic
Nitrogen (excess Nitrogen) affects various ecosystems (i.e. Marine Ecosystems)
is Eutrophication. Eutrophication, or commonly called hypertrophication is the
feedback of additional, or artificial Nitrogen containing compounds that enter
an ecosystem that under normal conditions would not. Substances such as
Nitrates will find their way into aquatic systems through sewage, or
agriculture byproducts (i.e. fertilizers).
Impacts
of Eutrophication
Before discussing the impacts of Eutrophication, I
would like to briefly explore some of the fundamental processes in relation to
marine ecology, focusing on the coral reefs.
Coral reefs are the most primary productive habitats
on earth, averaging between 2,500-5,000 grams of Carbon per meter square per
year globally; even compared to tropical wetlands under direct impacts of the
ITCZ, the net primary production is at least one third when compared to coral
reef biomes. Perhaps it’s the historical established mutualisms between corals
and other organisms that have lead coral reefs to be the most productive biome
on earth. Zooxanthellaes that fix Carbon or the Crustaceae which protect coral
from predation by various predators play an important role in the
sustainability of coral reefs.
As I mentioned above, Nitrogen is essential for all
living organisms. Same rule applies for marine organisms. Coral and
Phytoplanktonic organisms utilize Nitrogen that is fixed by Cyanobacteria,
which are considered to be “as old as life itself”. These cyanobacteria fix
carbon by photosynthesis and fix Nitrogen by Nitrogen Fixation, where for every
mole of Nitrogen gas they produce one mole of Ammonia, at the expense of 16 ATP
molecules; massive investment of energy.
Significance
As mentioned above, for one mole of Nitrogen, 16
molecules of ATP are required. This suggests that nature has made this process
extremely high energy demanding to perhaps establish a balance for the amount
of Nitrogen that enters a particular biome (Capone, 2001;
Capone & Montoya, 2001). What Eutrophication, as a result
of anthropogenic activities there is an increase phytoplankton population; they
grow better and rapid because there’s excess nutrition. This will result in
decomposition of phytoplankton which will decrease Oxygen levels. Decreased
Oxygen levels will result in hypoxic conditions (see picture below from NASA, the green regions
are near the estuaries and illustrate an average accumulation of Nitrogenous
compound input over time) which will have negative
impacts on marine biodiversity as well as an increase in the susceptibility to
invasive species (Bell, 1992). This will
cause a decline in coral reef because habitats such as the coral reefs are
nutrient-sensitive and require the lowest external inputs to trigger Eutrophication.
Hypoxic Zones (Visual Illustration)
Many sources of Eutrophication are illustrated in
the focus below. These are artificial sources of Nitrogen added to the marine environments.
Among these are:
Nitrogen compounds produced by cars
Discharge of untreated municipal sewage
Discharge of detergents and treated sewage
Runoff from streets, lawns, and construction lots
Discharge of untreated municipal sewage
Discharge of detergents and treated sewage
Runoff from streets, lawns, and construction lots
These external inputs of Nitrogen into the marine
biomes change the ecology and sustainability of these environments which in
turn will have an impact on species biodiversity and etc…
Nitrification
Nitrification is the process of ammonia conversion
into nitrite and nitrate (an overall diagram of the process below). Under aerobic conditions, prokaryotes carry out this
reaction; some fungi have been known to do this as well, but at much less
numbers compared to bacteria. The first step of the reaction, conversion of
ammonia into nitrite is done under aerobic conditions by prokaryotes known as
ammonia oxidizer. The first step of nitrification is done exclusively by
bacteria because they posses two enzymes, ammonia monooxygenase and
hydroxylamine oxidoreductase , which convert ammonia into an
intermediate, hydroxylamine (Rumer, Gupta,
& Kaiser, 2009). This process generates small
amounts of energy.
Dissimilar to Nitrogen fixation, which is carried
out by a broad spectrum of different microbes, ammonia oxidation is more
selective in terms of which microbes can carry the process out. Bacterial
genera such as Nitrosomonas, Nitrosopira, and Nitrosococcus are experts in
ammonia oxidation. Thus, not very many microbes are able to oxidize ammonia to
be used in step two of the process; conversion of nitrite to nitrate.
The second step of the process nitrification is
termed nitrite oxidation, where one mole of nitrite is oxidized via half a mole
of oxygen gas, or (just O), to generate one mole of nitrate. This process is
carried out by other exclusively selective prokaryotes known as nitrite-oxidizing
Bacteria. Both ammonia-oxidizers and nitrite-oxidizers function under
anaerobic conditions; the soil for instance, or in lakes and open-ocean
regions. These prokaryotes are also useful in maintaining a healthy environment
in the open-oceans. They facilitate the removal of toxic ammonium containing substances
excreted in fish urine and feces (Johnson et
al., 2010). Humans have, as always, have
figured out a way to take advantage of such processes.
Human Impacts
As mentioned above in the agriculture and Eutrophication
paragraphs, anthropogenic processing of nitrogen containing compounds will result
in anoxic zones in the ocean; especially at estuaries (Treusch et
al., 2005). One positive action done by
humans is take advantage of such processes. Synthetic (meaning man-made)
facilities help reduce or remove excess ammonium and prevent pollution of the waste
receiving waters. Image below is one example of such facilities near estuaries in bay area off the coast of SF.
Anammox
This process seems very important to me because it
has been associated to have direct impacts on nitrogen levels in the ocean and
the global nitrogen cycle; however, it is not generally discussed in many
websites that discuss the role of nitrogen. Including Wikipedia. In this part of the blog I would
like to explore the role of Anammox.
Generally, nitrification occurs and is carried out
under aerobic conditions; the presence of oxygen is essential. However,
relatively new discoveries have shown that ammonia oxidation can also occur in
anoxic environments (Strous et
al., 1999). A process called Anammox, which
stands for anaerobic ammonia oxidation, is carried out by a special type of
prokaryotes called Planctomycetes. The process utilizes special enzymes which
are still under study, to convert ammonia and nitrite into gaseous nitrogen and
water. Due to its nature, this process occurs in oceanic zones with low oxygen,
estuaries and freshwater lakes. Plant species in these areas are unable to grow
vastly and are few in numbers because the biousable nitrogen is sucked out of the
system (Kuypers et
al., 2005). Some argue that this is due to
denitrification (discussed below) rather than anammox. The overall chemical
reaction of Anammox is depicted below.
Denitrification
Denitrification is the process of regenerating
oxidized atmospheric nitrogen. It converts nitrate back into nitrogen gas and
therefore diminishes the available biousable nitrogen, returning it to the
atmosphere. Other gasses such as nitrous oxide (N2O) are the
intermediate by-products in the process of denitrification.
Dissimilar to nitrification discussed above,
denitrification is an anaerobic process and mostly occurs in soils and
sediments and anoxic zones in open oceans and lakes. Unlike, nitrification,
denitrification is carried out by a number of prokaryotes; recent studies
illustrate that denitrification can also be carried out by eukaryotes (Risgaard-Petersen et al., 2006). Denitrification
is actually a good thing! The overall reaction of denitrification is depicted
below.
Significance
Above, I discussed the impacts of nitrogen pollution
and excess nitrogen influx into the ocean – resulting in dead zones. Denitrification
is actually beneficial for oceanic creatures! It removes excess biousable
nitrogen from estuaries, impeding efflux of nitrogen to be taken up by algae.
N2O is considered a greenhouse gas and therefore
contributes to “air pollution”. I put air pollution in quotations because this
process is done in nature and therefore doesn’t really sound like pollution as
we know it. Regardless, it does react with the ozone and contributes to both
global warming and ozone layer destruction. It is however crucial to keep in
mind, that the amount of impact and input to greenhouse gasses is close to zero
compared to other sources of air pollution and greenhouse effect; such as
carbon dioxide.
Human
Impacts
Humans have constructed a facility that converts biousable
nitrogen back to its gaseous form to reduce Eutrophication. The Blue Pains
Wastewater Treatment Facility is a perfect example. This facility utilizes
methanol to enhance the efficiency the conversion nitrite or nitrate or ammonia
into N2. The images below are from the BPWTF.
Still today there's lots of research and effort towards reduction of nitrogen waste and ways to diminish its impacts on the biosphere.




.jpg)




.jpg)









